Biochemistry of Lyme
Disease: Borrelia burgdorferi Spirochete/Cyst
by Prof. Robert W. Bradford and Henry W. Allen
The Townsend Letter for Doctors and Patients, February-March 2006
http://www.townsendletter.com/FebMar2006/lyme0206.htm
Introduction
The history of science has presented some unusual twists and turns in man's
quest for knowledge. One researcher working quietly in one part of the world
may unwittingly be solving another researcher's problem in another location. As
we shall see in greater detail, government researchers and others, in an effort
to combat future bio-terrorist attacks, have unknowingly contributed greatly to
Lyme disease research. A discovery of great importance relating to a toxin
produced by the causative agent of Lyme disease, Borrelia burgdorferi,
has been linked to a similar toxin produced by the organism Clostridium
botulinum. The toxicity of these and other related substances is so great
that bio-terrorists have long considered using them in terrorist attacks
throughout the world. Anthrax and its spores are only one among many of such
candidate organisms. For this reason, US government scientists and others are
compelled to learn as much as possible about these highly dangerous toxins in
an effort to develop antagonists against their fatal action. It is remarkable
that the research to combat possible future bio-terrorist attacks may be
applied directly to therapeutic protocols for Lyme disease. A description of
these toxins and their biological activity is presented below, along with a
listing of therapeutic substances that may be applied in the treatment of Lyme
disease.
In 1982, the agent responsible for Lyme disease was discovered by Willy
Burgdorfer, who isolated spirochetes belonging to the genus Borrelia from the
mid-guts of ticks infecting deer, other wild animals, and dogs. Spirochetes are
spiral-shaped bacteria of very early origin in the evolutionary scheme. The
causative organism was named Borrelia burgdorferi (Bb), after its
discoverer. Since then, the number of reports of Lyme disease have increased so
dramatically that, today, Lyme disease is the most prevalent tick-borne illness
in the United States (carried by fleas, mites, mosquitoes, and ticks).
Lyme Disease Toxin
Because many of the symptoms of Lyme disease involve the nervous system, it
was speculated that the spirochete produced a toxin that disrupted normal nerve
function. Through the use of DNA manipulations and a database of known protein
toxin DNA sequences, a match was made with a selected Borrelia burgdorferi
(Bb) gene and a specific toxin in the database. Protein generated from this
cloned Bb gene was examined biochemically and found to have characteristics
similar to that of botulinum, the toxin of Clostridium botulinum, a zinc
endoproteinase.1
The toxin from Bb belongs to a family of toxic proteins known as "zinc
endoproteinases" or metalloproteases, and includes the toxin from the
organism causing tetanus as well as those from many other well-known infectious
diseases. The structures of this family of toxins are all very similar, as
determined by x-ray crystal analysis.2 They all contain zinc and
perform the same proteolytic function, namely, cleaving the chemical (covalent)
bond between two specific amino acids in a particular protein found in nerve
cells.3 The substrate for this enzyme is very large, implying that
any inhibitor of enzyme activity blocking the entry of the substrate into the
active site must also be very large.
One reason for learning the structure of the toxin (including the active site)
is to determine the geometry of this site, the exact positions of the atoms
that bind other atoms in the substrate. Knowing the arrangement of these atoms
permits the development of inhibitors of the toxin, substances that compete
with the normal substrate for active site occupancy.4
Action of Toxin
The action of botulinum (as well as the toxin from the Lyme spirochete) is
to prevent, through its action as a proteolytic enzyme, the release of the
neurotransmitter acetylcholine. Nerve endings may be associated with other
nerves or muscles (the neuromuscular junction). To understand this mechanism in
greater detail, consider the basic principles of nerve physiology described below.
Nerve Cells
A typical nerve cell consists of a long filament or axon, the terminal end
of which
lies in close proximity to another nerve cell. The space between them is known
as the synaptic cleft (synapse). One nerve cell communicates with another
through the release of a chemical substance known as a neurotransmitter held
within small sacs (vesicles) lying near the terminal end. An electrical pulse
travels the length of the axon and, when it reaches the nerve cell terminal,
causes the vesicles to rupture through the presynaptic membrane and discharge
the neurotransmitter into the synaptic cleft. The neurotransmitter is bound by
a protein (receptor) in the postsynaptic membrane of the adjoining nerve cell
causing, in turn, the transmission of an electrical pulse down the axon of the
second nerve cell. By this mechanism, nerve cells communicate with one another
through the action of a neurotransmitter. One such neurotransmitter is a simple organic substance known as acetycholine. (See Chart 1)

The structure of acetylcholine is shown by this formula:
CH3C(O)-O-CH2-CH2-N+(CH3)3
Mechanism of Neurotransmitter Release
Only recently has the mechanism of neurotransmitter release been understood at the molecular level. The proteins responsible for this highly detailed process have been isolated and characterized. Some parts of the puzzle are not as yet completely understood, for example, the process of membrane fusion. A study of the release of neurotransmitters from nerve endings has also revealed the mechanism of "switching," a process by which only one nerve among several in close proximity may be separately fired. This switching process is analogous to a similar process occurring in computers. Our brains work in a manner, in many ways, similar to that of computers. (See Chart 2.)
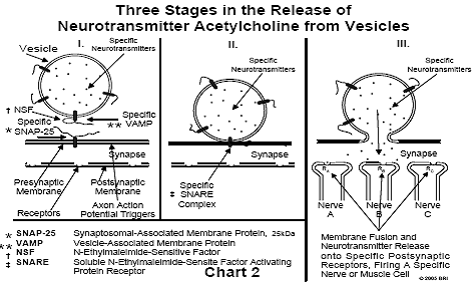
Each vesicle within a nerve ending contains only one type of
neurotransmitter. The vesicle containing a specific neurotransmitter (NT)
contains on its surface a specific protein designated VAMP (vesicle-associated
membrane protein). This protein is a member of a family of specific proteins,
differing only in the sequence of amino acids forming a chain extending from
the protein. If the NT is designated NTA, the VAMP found in the membrane of the
vesicle containing NTA, will always be VAMPA. In other words, a specific
neurotransmitter is always associated in the vesicle with a specific type of
VAMP. Finding another type of VAMP – for example, VAMPB – on the surface of a
vesicle containing NTA will never occur. The difference between VAMPA and VAMPB
lies only in the sequence of amino acids in the peptide (protein chain)
extending from the protein.5
During the random motion of vesicles in the region of a nerve ending, some
encounter another protein embedded in the presynaptic membrane, designated
SNAP-25 (synaptosomal-associated membrane protein). All SNAP-25 proteins belong
to a family of similar proteins, differing only in the amino acid sequences of
two peptides extending from the protein. A particular member of this family
may, for example, be designated (SNAP-25)A. If a vesicle bearing on its surface
the protein VAMPA encounters the protein (SNAP-25)A lying in the presynaptic
membrane, the three peptides (two from SNAP-25 and one from VAMP) rapidly
intertwine and automatically form a triple helix, which twists in a manner
similar to a "twist-tie" used on bread wrappers (ATP-driven). The
structure of this peptide triple helix is similar to the triple helix found in
collagen (a).5
The result of the twisting action is to draw the vesicle close to the surface
of the
presynaptic membrane. When the membrane of the vesicle contacts the presynaptic
membrane, the two membranes automatically fuse, resulting in the vesicle
contents (containing NTA) emptying into the synapse. The membrane flattens out
and the VAMP/SNAP-25 proteins (the SNARE complex) are recycled.6 (See Chart 2)
NSF Protein
A third protein linked to the VAMP/SNAP-25 complex is
N-ethylmaleimide-sensitive factor (NSF). N-ethylmaleimide is simply a chemical
reagent used by biochemical researchers (not a normal body metabolite), capable
of attaching acetyl groups [CH3C(O)-] to sulfhydryl groups (-SH) as
found in the amino acid cysteine, a constituent of many proteins. The protein
NSF is "sensitive" to this reagent (binds acetyl groups when exposed
to the reagent), indicating that its surface is rich in sulfhydryl groups. This
observation gives a hint about the activity of NSF, an agent that holds
together two other proteins (VAMP and SNAP-25). Sulfhydryl groups are normally
used to bind two proteins together (cross-linking) or to bind different parts
of a single protein to each other. This is accomplished by the elimination of
two hydrogens (-H) from two sulfhydryl groups (-SH) (usually by a single atom
of oxygen, thereby forming water), resulting in a disulfide linkage (-S-S-).
For this reason, NSF is believed to function as a link between VAMP and
SNAP-25, forming a single rigid unit.5 (See
Chart 1)
Specificity of Nerve Firing
If a vesicle having VAMPA on its surface encounters a (SNAP-25)B (or any
type other than A), no intertwining of the peptides will occur, the vesicle
will not contact the presynaptic membrane and, consequently, no
neurotransmitter will be released.
The NTA, released into the synapse, almost immediately contacts a receptor (RA)
in the postsynaptic membrane capable of binding this neurotransmitter. If this
receptor is found in nerve A (see Chart 2), this nerve only is fired (i.e.,
develops an action potential that travels down the axon). Any nerve ending in
close proximity not carrying RA in its postsynaptic membrane will not be
activated. If NTB is released into the synapse, only those nerve endings
carrying RB will be activated. By synthesizing large amounts of vesicles
containing NTA and simultaneously synthesizing an equal number of (SNAP-
25)A, the corresponding type of nerve is activated.5
Dietary Supplements in Lyme Disease
One of the known actions of the Lyme spirochete toxin is to diminish the
release and availability of the neurotransmitter acetylcholine, a simple
organic compound (see above for chemical structure). This substance is
biosynthesized by the body as required in nerve activation and transmission.
Supplementation by the precursors of acetylcholine synthesis would be of value
to Lyme patients since they have a deficiency of this substance. (See Listing 1)
Listing 1: Dietary Supplements Increasing Acetylcholine Synthesis Improving Neurologic Function
- Phosphatidylcholine (Lecithin)Acetyl-L-Carnitine
- Vitamin B5 (Pantothenic Acid)
- Vitamin B6 (Pyridoxine)
- Vitamin C (Ascorbic Acid)
- Lysine (Amino Acid)
- S-Adenosylmethionine (SAM) (Sulfur-bound Adenosyl Methionine)
If the inhibition of acetylcholine release were total, Lyme
patients and those suffering from food poisoning would not be able to move;
they would be completely paralyzed. Since the blockage is only partial, any
increase in the amount of available neurotransmitter would benefit anyone
experiencing neurotransmitter blockage. For this reason, dietary supplements increasing
the amount of available acetylcholine have been shown to benefit Lyme patients.
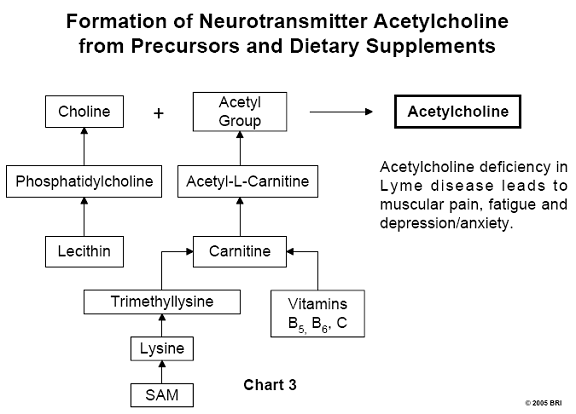
Acetylcholine Formation
In Chart 3, we can see phopsphatidylcholine is a constituent
of lecithin, a well-
known dietary supplement. Acetylcholine is simply choline to which an acetyl
group (CH3CO-) has been attached. Lecithin is the source of choline,
and acetyl-L-carnitine (ALC) is the source of the acetyl group. Carnitine is
synthesized by the body and requires several factors, including the amino acid
lysine and vitamin C (ascorbic acid). The supplement known as SAM
(S-adenosylmethionine) supplies methyl groups (CH3-) to lysine,
forming trimethyllysine. This compound is further processed, requiring
additional vitamin C, resulting in carnitine that supplies the necessary acetyl
group.8,9
History of Lyme and Related Spirochetal Diseases
The discovery by Burgdorfer that Lyme disease was caused by a spirochete
placed it in a category of other diseases known to be caused by spirochetes. An
example of such a disease is syphilis, the scourge of Europe for hundreds of
years. Arsenic and some of its compounds had been known for quite some time as
a highly successful and popular means of fatally poisoning someone (remember
the King in Shakespeare's Hamlet). Following the discovery of the Germ Theory
of Disease by Louis Pasteur (1822–1895), it was theorized that, if arsenic was
toxic enough to kill, it may also be effective in killing the organisms that
cause disease. In the early 1900s, the German chemist-physician Paul Ehrlich
(1854–1915) developed a chemical treatment for syphilis. By using a
"shotgun" approach of trying hundreds of compounds in an effort to
find one that worked, Ehrlich discovered what became known as Salvarsan or
"606" after 606 compounds had been tested. Salvarsan is an organic
compound of arsenic and may be highly toxic if not properly used. For his
monumental discovery, Ehrlich was awarded the Nobel Prize in 1908. Salvarsan
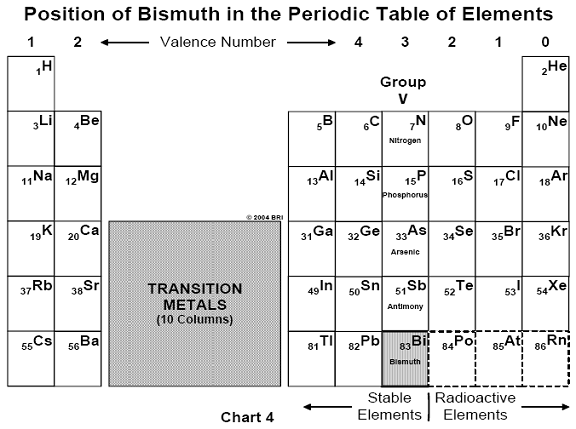
may be considered the first man-made antibiotic.26 Arsenic belongs
to that column in the periodic table of chemical elements known as the
"Group V elements," which also include phosphorus, antimony and
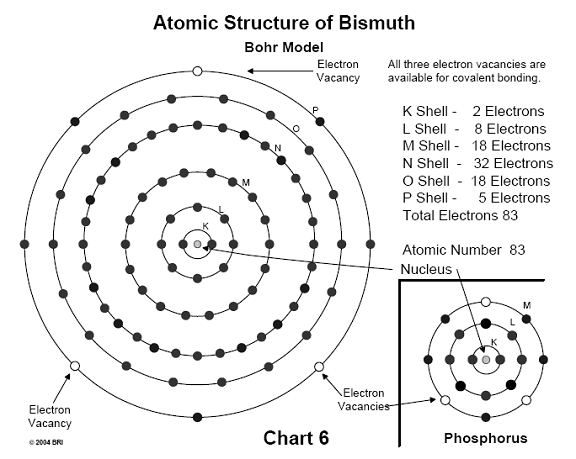
bismuth. (See Chart 4).
Following the success of Salvarsan as a treatment for
syphilis, other compounds of antimony and bismuth were also prepared and tried
against spirochetes. Examples of these compounds include bismuth subcitrate,
bismuth subsalicylate (Pepto-Bismol), bismuth subgallate, and many others. An
example of an antimony-containing antibiotic is Pentostam (an antimonial,
antimony sodium gluconate).27,28
A biological molecule known as ATP (adenosine triphosphate) supplies energy to
biological systems through the high energy bonds found in a chain of three
terminal phosphate groups. One of the mechanisms by which arsenic exerts its
toxic effect is the substitution of phosphorus by arsenic in ATP, since both
arsenic and phosphorus lie in the same column of the periodic table of chemical
elements and have similar chemistry. (See Chart 5).
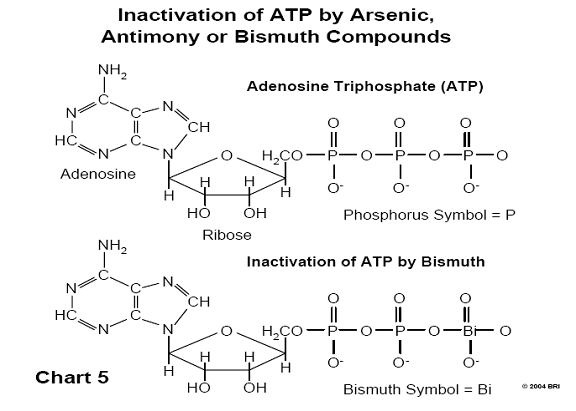
When this substitution occurs, the molecule experiences immediate hydrolysis, breaks down, and no longer functions as a source of energy for the cell. Both antimony and bismuth are also found in this column of the periodic table (Group V). 29,30 (See Chart 6)
What may be the first case of Lyme disease was noted about 1974 in a 14-year old boy, taken to the hospital with extreme pains in the muscles of his legs and unable to walk. This case, coupled with other pertinent facts related to the boy and a highly classified US government laboratory conducting research on contagious animal diseases in this same area, is suggestive of a link between these two events. The government laboratory alluded to is found on Plum Island, just north of Long Island, NY, and south of Lyme, Connecticut. Because of its secret nature, access to the island was only by ferry boat and restricted to the government workers employed there. The 14-year old boy lived near the ferry boat dock. Although not providing proof, these considerations are highly indicative of a possible link between this research laboratory and the subsequent outbreak in 1975 of an unknown disease involving juveniles in the same area of Lyme, Connecticut.32 A condensed form of the history of Lyme disease is shown in Listing 2.23
Listing 2: History of Lyme Disease
1900
Effective antisyphilitic, Salvarsan, (syphilis, a spirochete disease)
discovered by Paul Ehrlich, MD.
1908
Ehrlich awarded Nobel Prize for the arsenic-containing compound to treat
syphilis.
1952/2004
Highly classified US Government animal disease research
laboratory, Plum Island, in close proximity to Lyme,
CT.
1974
First Lyme symptoms, 14-year old boy, Lyme, CT.
1975
Lyme disease first recognized by Allen Steere, MD, in Lyme, CT.
1982
The causative Lyme spirochete was discovered by Dr. Willy Burgdorfer.
1983
Borrelia burgdorferi was named after Dr. Willy Burgdorfer.
2003
American Biologics' Bradford Variable Projection Microscope
(BVPM) images of Lyme spirochete and cyst forms.
2004
Dr. Robert Bradford, through the Bradford Research Institute (BRI), an
independent research entity, funded by American Biologics, is the developer of
Bismacine,TM a chemical compound of bismuth. This formulation has shown to be
effective at the Ingles Hospital against the
spirochete and cyst forms of the Lyme organism.
© 2004 BRI
Etiology and Difficulty of Treatment
The first step in being able to treat any disease is to learn the cause
(etiology) of that disease. Once the cause of Lyme disease was known, it seemed
that a treatment modality would soon follow and the problem would be solved.
Unfortunately, as history has shown, this was not to be the case. As more was
learned about the causative agent, namely, the spirochete Borrelia
burgdorferi, it became obvious that this organism was unlike any that had
been previously studied. It is one of the largest of spirochetes (0.25 x 25 µ)
Spirochetes in general are difficult to treat for several reasons: They have
the ability to burrow into or between cells and hide, gaining protection from
the immune system. Both Bb and Treponema pallidum, the causative agent
for syphilis, have highly unusual outer membranes, and the molecular
architecture of these membranes is responsible for their ability to cause
persistent infection.
Bb also has a three-layer cell wall, helping to determine the spiral shape of
the spirochete. This distinctive cell wall resembles those of Gram-negative
bacteria, although Bb does not stain Gram-negative but is stained by silver
stains (containing silver nitrate). This characteristic may be related to the
purported treatment of Lyme disease by colloidal silver.33
Another unusual structural feature is a single flagella, attached to each end
of the spirochete, running the length of the organism and surrounded by it.
This feature is significant in relation to immune protection, since most
bacterial flagella are highly antigenic. Still another difference in Bb
structural architecture is a clear gel-like coating surrounding the bacteria,
giving it protection from the immune system.31 (See Chart 7)
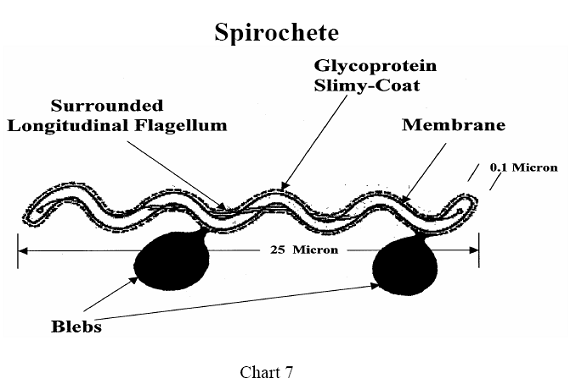
The DNA of Bb is arranged in a different manner than in
other bacteria, lying along the inside of the inner membrane, and resembling a
net just under the skin. The bacteria replicates specific genes, inserts them
into its own cell wall and then pinches off that part of the cell membrane,
releasing it into the surrounding medium. This fragment of the spirochete
membrane with incorporated DNA is known as a "bleb." It is not
understood why this strange event occurs or what advantage it gives the
organism but some studies suggest that the function of blebs is to bind IgM
antibodies, thereby protecting the organism from the immune system.33
Bb is one of the most immuno-suppressive infectious agent, affecting cellular
immunity, humoral immunity, and natural killer (NK) cell population.24, 25
The spirochete is typically observed in the Bradford Peripheral Blood
Assessment (BPBA) utilizing the Bradford Variable Projection Microscope (BVPM)
in three different forms.23
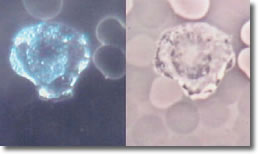
I. Normal spiral form of spirochete, length of approximately 25 µ with evenly spaced blebs along its membrane. (See Photo 1)
Photo 1
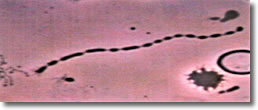
Darkfield-Phase
10,000X
II. The elongated bleb form described above, by doubling back on itself, forms a circle of blebs. (See Photo 2)
Photo 2

Darkfield-Phase
10,000X
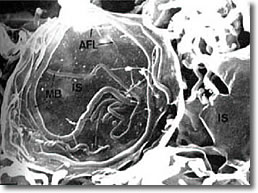
III. The elongated form doubles back on itself, forming close-packed multiple clusters of figure 8s (convolutions), typically observed inside a B-cell, but may been seen isolated. (See Photo 3)
Photo 3
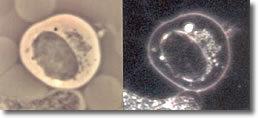
Phase
18,000X
IV. Cyst forms developed inside a B-cell, without the clustered spiral form of the spirochete. (See Photo 4)
Photo 4
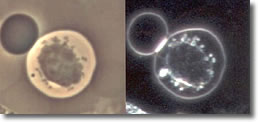
Phase-Darkfield
10,000X
V. Cyst forms developed inside a B-cell with clustered spiral form of spirochetesee. (See Photo 4A)23
Photo 4A
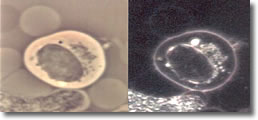
Phase-Darkfield
10,000X
VI. Cyst forms inside a basophil. (See Photo 5)
Photo 5
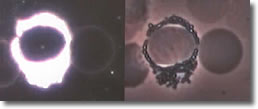
Darkfield-Phase
12,000X
VII. Cyst forms inside an eosinophil. (See Photo 6)
Photo 6
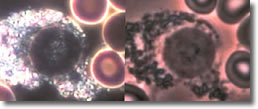
Darkfield-Phase
10,000X
VIII. Scanning electron microscopy of blebs on spirochete membrane. (See Photo 7)
Photo 7
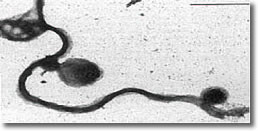
Electron Microscopy
Bb deposits cysts inside eosinophil segments with the immune response similar to parasite infection, resulting in increased EOC. Photo 8 shows an infected EOC and a normal EOC.
Photo 8
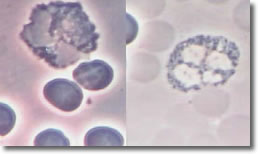
Infected
Normal
Phase Phase
EOC
10,000X
Bb deposits cysts inside basophil segments. Photo 9 shows an infected basophil and a normal basophil.
Photo 9
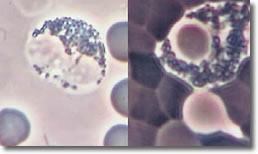
Normal Infected
Phase Darkfield
Basophil
10,000X
The PMNs, after a finite period of time, will start to recognize the deposited cysts in the WBCs and put their energy into destroying the cysts. In this process, the PMNs stop normal cytoplasmic streaming with a resultant increase in bacteria count. (See Photo 10)
Photo 10
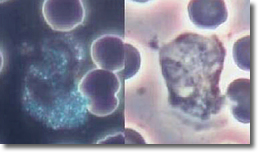
Normal Normal
Darkfield Phase
Neutrophil
10,000X
Photo 11 shows a non-infected PMN cytoplasmic streaming activity.
Photo 11
Infected Infected
Darkfield Phase
Neutrophill
10,000X
The cell division time of Bb is very long compared to other
bacteria. A typical cell wall reproduction time for Streptococcus or
Staphylococcus is less than 20 minutes, while the total reproduction time of Bb
is from 12-24 hours. Most antibiotics inhibit the formation of cell walls and
are effective only when the bacteria are dividing with the formation of new
cell wall. With the slow replication time of Bb, an antibiotic would have to be
present 24 hours a day for one year and six months to be present during the
cell wall reproduction period.33
There are basically two mechanisms by which Bb can survive within the host and
remain for long periods of time, unknown by the victim. Because of these
processes, a person infected by Bb can remain unsymptomatic for long periods of
time and then suddenly, without warning, begin to experience symptoms once
again. One of these mechanisms involves the invasion of tissues by the
spirochete. The tip of the organism has the ability to bind to cells, spin and
twirl until it stimulates the cells own enzymes to digest a part of the
membrane, finally allowing entry. Once inside, the spirochete results in either
the death of the cell or takes up residency within. It may lie dormant for
years, protected from both the immune system and the action of antibiotics.
Experiments have shown that, if a culture of Bb is placed under conditions of
nutrient deprivation or starvation, it senses that it cannot survive in a
metabolically active state and generates what are known as "cysts" or
small sacs attached to the organism by slender threads. Cysts contain immature
spirochetes in a metabolically inactive form. Eventually, they break off from
the parent body and either remain lodged in tissues or enter the blood where
they are sensed as foreign antigens by eosinophils (a type of WBC) and
phagocytized. Eosinophils release granules of positively charged basic protein
that attach to the normally negative surface of cells. They attempt to destroy
the invading foreign bodies (cysts) but have little success.33 (See
Photo 12.)
Photo 12: Scanning electron microscopy of the spirochete cyst form23
Lymphocyte Invasion by Bb
When a spirochete attacks a B-cell, it attaches the tip to the surface,
spins and twirls until it enters, then multiplies inside until the B-cell
bursts. Some spirochets become coated with fragments of B-cell membrane and
escape detection by the immune system by masquerading as a B-cell. Most of the
antigenic proteins in Bb (those in other bacteria mark the microorganism for
destruction by the immune system) are found on the inside of the inner membrane
where they cannot contact those WBC that detect invaders.33
Bb Surface Antigens
Experiments have shown that Bb can rather quickly change surface antigens
so that antibodies made against one strain are effective in killing that
strain, but a second strain having different surface antigens will take up
residence in a different tissue where it escapes detection and survives. For
these reasons and others, it becomes apparent that this particular spirochete
has evolved disguises and biological techniques to guarantee its survival and
thwart any attempts to circumvent it.33 (See Listing 3.)
Listing 3: Distinguishing Characteristics of Borrelia burgdorfer
Internal Flagella
Glycoprotein Coat
DNA Net Arrangement
Bleb Formation
Prolonged Replication Time
Cellular Invasion Ability
Cyst Formation
Destruction of B-Cells
Camouflage as B-Cell
Internal Antigenic Proteins
Surface Antigen Transformation
Spiral Shape
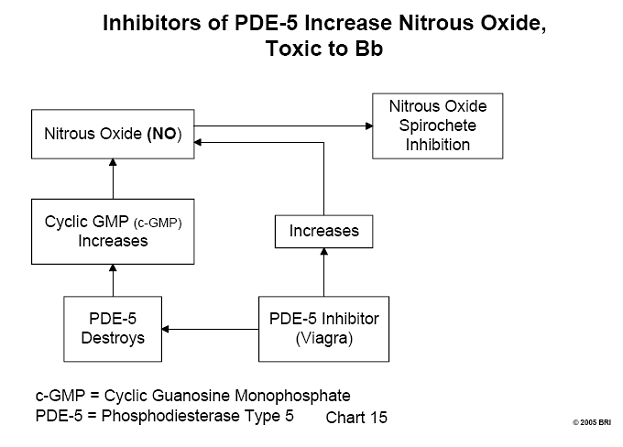
Nitrous Oxide (NO), A Potential Lyme Therapeutic Agent
Nitrous oxide (chemical formula NO) is a gas, at one time commonly used as an
anesthetic (laughing gas). In more recent times, the biochemical activity of NO
has been related to the relaxation of the small muscle fibers in the walls of
blood vessels. They serve to either relax or constrict the flow of blood
passing through those vessels. The mechanism of NO bioactivity has also been
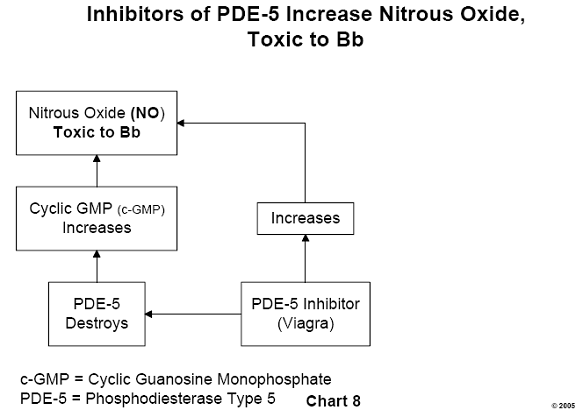
learned; this involves the substance c-GMP (cyclic guanosine monophosphate).
The amount of c-GMP at any time is regulated by the enzyme, phosphodiesterase type
5 (PDE-5), having the capacity to destroy it. c- GMP fits into a cavity on the
surface of PDE-5, the "active site" of this enzyme. Any other
substance capable of being bound by the active site of PDE-5 inhibits the
activity of the enzyme by blocking the entry of c-GMP, thus allowing a greater
survival of c-GMP. To summarize, any inhibitor of PDE-5 allows an increase in
the amount of available c-GMP and consequent relaxation of blood vessels,
permitting a greater flow of blood through those vessels.10
It has been demonstrated that NO is toxic to Borrelia burgdorferi, the
causative organism of Lyme disease.11 Therefore, any inhibitor of PDE-5 is a potential therapeutic agent for Lyme disease. Inhibitors of PDE-5 in common use today are the drugs sildenafil (more commonly known as Viagra), Levitra, and Cialis. Whether these drugs act therapeutically against the Lyme spirochete has not been demonstrated clinically and remains unknown. (See Chart 8.)
Inhibitors of the Lyme Spirochete Toxin
A large amount of work is being conducted today in an effort to uncover more
inhibitors of the Lyme spirochete toxin. One known inhibitor of toxin activity
is the substance glycyrrhizic acid (GA), the active principle of licorice root,
used in Oriental medicine for thousands of years.12 GA is also the
active principle of the American Biologics product, Biorizin™. The molecular
structure of GA includes a steroid with large bulky substituents. Being a large
molecule, GA is capable of binding into the active site of the toxin, thereby
blocking the normal substrate, two adjacent amino acids in the protein SNAP-25.
(See Chart 8 and Chart 9)
A second inhibitor of Lyme (botulinum-like) toxin is the dipeptide, glutamylglutamate (Glu-Glu), consisting of two glutamic acids bound together as a dipeptide.13 The tripeptide Glu-Glu-Glu also inhibits botulinum.13 These substances are inhibitors because of their similarity to the amino acid pair, asparagine- phenylalanine, the normal substrate of botulinum. Although being bound by the toxin's active site, the toxin is unable to cleave the Glu-Glu linkage. (See Chart 8 and Listing 4.)
Listing 4: Inhibitors of Borrelia burgdorferi (Bb) and its Toxin
Inhibitor
Glycyrrhizic Acid (Licorice Root) Biorizin™
Glutamylglutamate (Glu-Glu Dipeptide)
Nitrous Oxide (NO) (Arginine Stimulates Production)
Bismacine™
Chromocine™
Silver Ion
Inhibits
Toxin
Toxin
Bb
Bb
Bb
Bb
© 2005 BRI
Lyme Spirochete Binds to Hostal Tissue
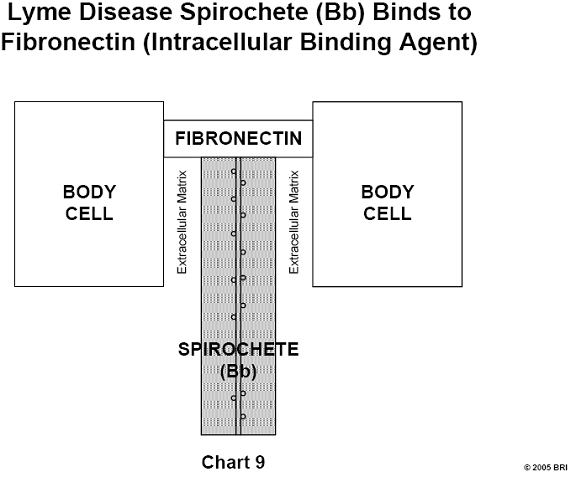
A specific protein (BBK32) has been isolated from the Lyme spirochete Bb and
has been shown to bind fibronectin, the universal cellular binding agent. This
discovery may be highly significant in relation to the known ability of Bb to
become deeply imbedded and hide in most hostal tissue.14 (See Chart 9)
Structure Determination of Bb Outer Surface Proteins
The structures of two outer surface proteins (OspA and OspC) have been
determined by x-ray crystal analysis to a resolution of 2.5 A. OspA has been
found to be very different from OspC relative to the arrangement of alpha
helices and other folding of the protein.15
Structure Determination of Botulinum Complexed with SNAP-25
Botulinum, a neurotoxin produced by the organism Clostridium botulinum
is one of the agents responsible for food poisoning. A similar toxin is
produced by the Lyme causative organism Borrelia burgdorferi. The
detailed structure of botulinum complexed with its substrate, SNAP-25, may lead
to the development of inhibitors of complex formation.16 (See Chart 10).
Major Diseases Linked to Lyme Spirochete
Lyme Spirochete Found in the Brain of MS Patients
The causative organism of Lyme disease, Borrelia burgdorferi, has
been found in the brains of many victims of multiple sclerosis (MS). The
antibiotics minocycline, tinidazole, and hydroxychloroquine are reportedly
capable of destroying both the spirochetal and cyst form of Bb. Because of this
apparent correlation, it is proposed that double-blind clinical trials be
performed to confirm this finding.17 (See Listing 5)
Listing 5: Lyme Disease Linked to Four Major Diseases
Multiple Sclerosis, Alzheimer's, Systemic Scleroderma and Arthritis
ALZHEIMER'S
The spirochete Borrelia burgdorferi has been found in the brain of
many Alzheimer patients. Also in the brain, antigens and genes of Bb have been
co-localized with beta-amyloid deposits.
MULTIPLE SCLEROSIS
The spirochete Borrelia burgdorferi (Bb) has been found in the brain
of many multiple sclerosis (MS) patients along with amyloid deposits. MS has
been linked to Lyme disease both seasonally and by location.
SYSTEMIC SCLERODERMA
The spirochete Borrelia burgdorferi has been found in the blood in
systemic scleroderma. Treatment with antibiotics effective against Bb returned
the skin to normal.
LYME-INDUCED ARTHRITIS
Only certain strains of Bb are capable of causing the symptoms of
arthritis.
© 2005 BRI
Lyme Spirochete Found in the Brain of Alzheimer Patients
Spirochetes found in the brain of many Alzheimer disease (AD) patients were
positively identified as Borrelia burgdorderi, the causative organism of
Lyme disease. Borrelia antigens and genes were also co-localized with
beta-amyloid deposits in these AD cases.18 (See Listing 5, above)
Lyme Spirochete Linked to Systemic Scleroderma
A patient confirmed to have systemic scleroderma was also shown to be infected
with the Lyme spirochete, Bb. Treatment with antibiotics known to be effective
against Bb returned the skin of this patient to normal within a few weeks.19
(See Listing 5, above)
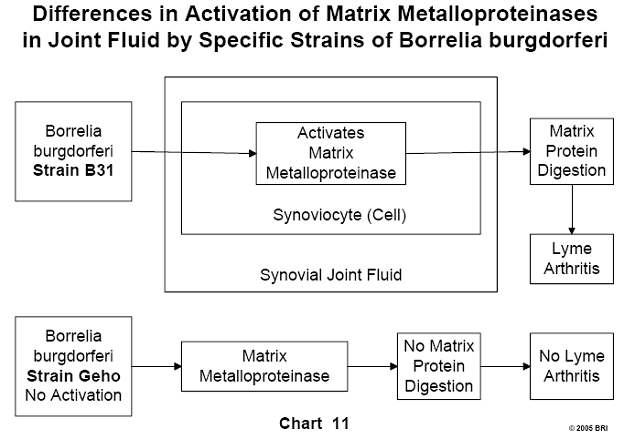
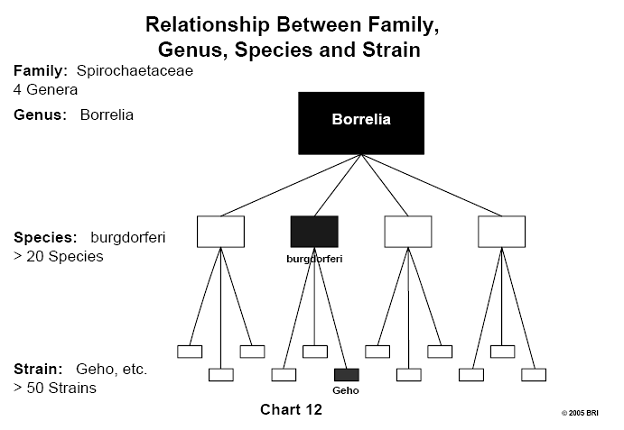
Lyme-Induced Arthritis Linked to Various Strains of Bb
It has been noted clinically that some Lyme-induced arthritis patients are
affected by the disease to different degrees. A laboratory study demonstrated
that different strains of Bb were capable of activating to various degrees a
particular enzyme (matrix metalloproteinase) found in human synoviocytes. These
cells are found in the synovial fluid of joints and form some of the substances
found in this fluid. Matrix metalloproteinases are proteolytic enzymes capable
of degrading most of the proteins in the extracellular matrix. Different
strains of Bb activate these proteases to varying degrees, explaining
variations seen clinically in the severity of Lyme-induced arthritis. To date,
more than 50 strains of Bb have been identified.20 (See Chart 11 and Chart 12).
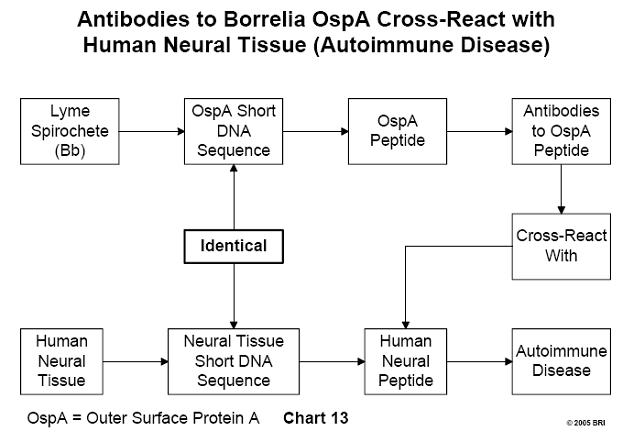
Similarity Between DNA Sequences of Brain Tissue and Bb OspA
DNA sequences of Bb outer surface protein A (OspA) compared with a data
bank of DNA sequences of human neural tissue yielded three sequences that were
identical. The three corresponding Bb peptides were synthesized, and antibodies
were induced against them. The antibodies cross-reacted with human neural
tissues.
These findings imply that antibodies developed by Lyme disease patients against
OspA will also bind to their own neural tissue, representing a form of
autoimmune disease in which a person's immune system attacks his own tissues.21
(See Chart 13)
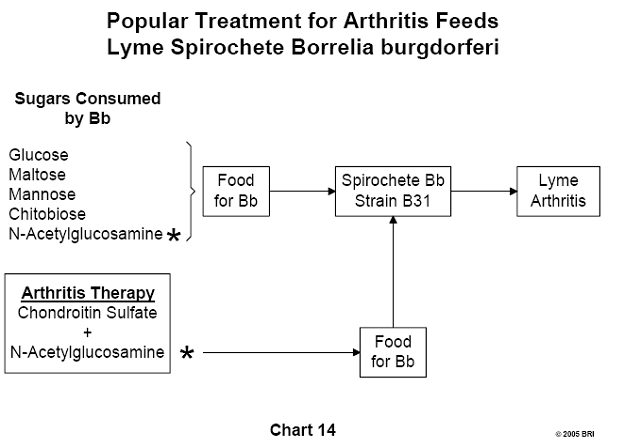
Carbohydrates Consumed by Lyme Spirochete
An effort to determine which carbohydrates Bb consumes revealed that the
organism utilizes the monosaccharides glucose, mannose and N-acetylglucosamine,
as well as the disaccharides maltose and chitobiose. A popular treatment for
arthritis includes the administration of chondroitin sulfate and
N-acetylglucosamine. If the arthritis is Lyme-induced, N-acetylglucosamine is
contraindicated.22 (See Chart 14)
See Chart 15: Inhibitors of PDE-5 Increase Nitrous Oxide, Toxic to Bb
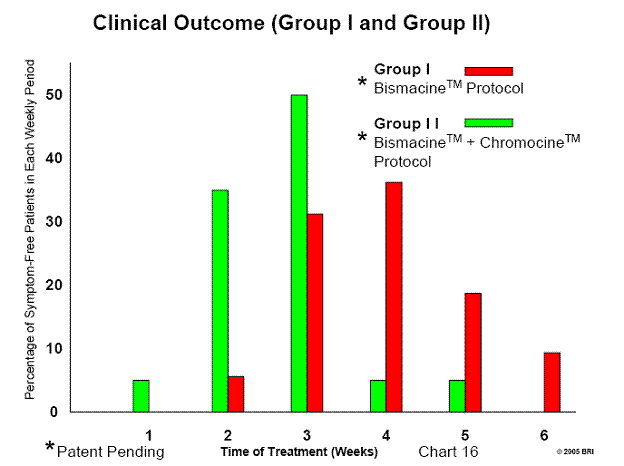
Listing 6: Bradford Research Institute/Ingles Hospital Preliminary Clinical Outcome
Group I: 50 Ingles Hospital patients, Bismacine™ therapy, 100% favorable
response
Group II:20 Ingles Hospital patients, Bismacine™ with Chromocine™ therapy
Reoccurrence - 3 patients (4%) in Group I
Bismacine™ with Chromocine™, our most efficacious therapy to date.
© 2005 BRI
Listing 7: Clinical Outcome Data (Group I)
Preliminary Data
Treatment Dates January 2004 through April 2005 (14 months)
Treatment Program BRI Bismuth Protocol
Number of Patients 55 (Male - 21 Female - 34)
Age of Patients 18 years to 76 years
Patient Response Acute Herxheimer reactions (10 days to 2 weeks)
Duration of Treatment 2 weeks to 6 weeks (in-patient)
Results
Duration of Treatment
2nd week 3 Patients - 5.6%
3rd week 19 Patients - 30.9%
4th week 20 Patients - 36.4%
5th week 10 Patients - 18.2%
6th week 5 Patients - 9.0%
© 2005 BRI
Listing 8: Clinical Outcome Data (Group II)
Preliminary Data
Treatment Date April 2005 through May 2005
Treatment Program Bismacine™ plus Chromocine™ protocol
Number of Patients 20 (Average patients/month - 3.9)
Male - 15
Female - 5
Age of Patients 17 years to 92 years
Patient's Response Minimal to no Herxheimer reactions
Duration of Treatment 1 to 5 weeks (in-patient)
1st week 1 Patient 5.0%
2nd week 7 Patients 35.0%
3rd week 10 Patients 50.0%
4th week 1 Patient 5.0%
5th week 1 Patient 5.0%
See Chart 16: Clinical Outcomes
Conclusion
Bb is one of the most immunosuppressive infectious agents known and, as a
result, many secondary infectious agents are found along with Bb, including
fungus, virus, bacteria, and mycoplasma. Clinically, these concurrent agents
and their mechanisms are in themselves immunosuppressive and must be
functionally assessed, diagnosed, and treated in order to achieve an effective
Lyme disease program.
References
All references beginning with http:// are internet addresses.
1. Cartwright MJ, Martin SE, Donta ST. A novel neurotoxin (Bb Tox 1) of
Borrelia burgdorferi. Abstracts: General Meeting of the American Society for
Microbiology,1999:54. http://www.lyme.org/conferences/99_abstract.html
2. http://www.stormingmedia.us/18/1815/A181553.html
3. Schmidt JJ, Stafford RG. Fluorigenic substrates for the protease activities
of
botulinum neurotoxins, serotypes A, B, and F. Appl Environmental Microbiol.
2003;69:297-303.
4. http://www.medicalnewstoday.com/index.php?newsid=8150
5. http://www.neuro.wustl.edu/neuromuscular/pathol/snare.htm
6. http://ajpcell.physiology.org/egi/content/full/285/2/C237#FIG1
7. http://www.pasteur.fr/recherche/borrelia/Borrelia_burgdorferi.html
8. Vaz FM, Wanders R. Carnitine biosynthesis in mammals. Biochem J.
2002;361:417-29.
9. http://www.orbit6.com/cognition/neurotr1.htm
10. http://www.physiciansselect.com/L-arginine-information.htm
11. http://www2.lymenet.org/domino/nl.nsf/0/9e85f54e56d31dc5852565e30017f60f?
OpenDocument (2/6/06: Link does not work.)
12. Hayden J, Pires J, Roy S et al.. Discovery and design of novel inhibitors
of
botulinus neurotoxin A: targeted "hinge" peptide libraries. J Appl Toxicol. 2003;23:1-7.
13. http://jmedchemdef.org/archives/CBMTSIII/cbmts3-38.pdf
14. Raibaud S, Schwarz-Linek U, Kim JH et al. Borrelia burgdorferi
binds
fibronectin through a tandem beta zipper – a common mechanism. J Biol Chem.
2005 Feb 28 (E-Published ahead of print).
15. Eicken C, Sharma V, Klabunde T et al. Crystal structure of Lyme disease
antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem.
2001; 276:10010-5.
16. Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum
neurotoxin serotype A. Nature. 2004;432:925-9.
17. Fritzsche M., Chronic lyme borreliosis at the root of multiple sclerosis –
is a cure with antibiotics attainable? Med Hypotheses. 2005;64:438-48.
18. Miklossy J, Khalili K, Gern L et al. Borrelia burgdorferi persists
in the brain in chronic lyme neuroborreliosis and may be associated with
Alzheimer disease. J Alzheimers Dis. 2004;6:639-49.
19. Wackernagel A, Bergmann AR, Aberer E. Acute exacerbation of systemic
scleroderma in Borrelia burgdorferi infection. J Eur Acad Dermatol
Venereol.
2005;19:93-6.
20. Singh SK, Morbach H, Nanki T et al. Differential expression of matrix
metalloproteinases and cyclooxygenases in synovial cells exposed to borrelia
burgdorferi. Inflamm Res. 2004;53:689-96.
21. Alaedini A, Latov N. Antibodies against OspA epitopes of borrelia
burgdorferi cross-react with neural tissue. J Neuroimmunol.
2005;159:192-5.
22. von Lackum K, Stevenson B. Carbohydrate utilization by the lyme borreliosis
spirochete, borrelia burgdorferi. FEMS Microbiol Lett.
2005;243:173-9.
23. Bradford RW, Allen HW. Lyme Disease, Potential Plague of the Twenty-First
Century. Chula Vista, California: Bradford Research Institute; 2004.
24. Zajkowska JM, Hermanowska-Szpakowicz T. Subpopulations of the peripheral
lymphocytes in the early clinical forms of lyme disease. Med Sci Monit.
2000;6:278-84.
25. http://www.anapsid.org/lyme/strickerpanel.html
26. http://www.dailymirror.lk/inside/junior/020530.html
27. http://www.intox.org/databank/documents/sodstib/ukpid80.htm (2/6/06:
Link does not work.)
28. Sox TE, Olson CA. Binding and killing of bacteria by bismuth subsalicylate.
Antimicrob Agents Chemother. 1989;33:2075-82.
29. http://www.atsdr.cdc.gov/HEC/CSEM/arsenic/physiologic_effects.html
30. http://www.treedictionary.com/DICT2003/shigo/CHEM.html
31. http://www.lymenet.de/literatur/Microbiology.htm
32. Carroll MC. Lab 257: The Disturbing Story of the Government's Secret
Plum Island Germ Laboratory. New York: William
Morrow Publishing Co.; 2004.
33. Grier, T. The Complexities of Lyme Disease, from: Lyme Disease Survival
Manual, 1997.